Introduction
The field of materials science has seen some exciting developments lately, especially with electronic band structures. Researchers are exploring new materials like graphene, which is just one atom thick, and making big strides. One cool update is how scientists are now able to tweak these materials' properties on the fly. They use things like electric fields or stretching the material to change how it behaves. This means we might soon have electronics that you can adjust however you need, making them more versatile than ever.
What Are Electronic Band Structures?
Electronic band structures describe how electrons are distributed in materials. These structures depict the allowed and forbidden energy levels of electrons in solids. Think of it as a hotel where electrons check into rooms (energy levels) that determine how the material behaves—whether it’s conducting electricity or blocking it.
Key Concepts in Band Theory
Energy Bands and Band Gaps
In solid materials, electrons can occupy certain energy bands. The band gap is a crucial concept here—it's the energy difference between the top of the electron-occupied valence band and the bottom of the empty conduction band. Materials with large band gaps are generally insulators, while those with smaller band gaps are conductors or semiconductors.
Valence Bands and Conduction Bands
The valence band is where the electrons reside at zero temperature, and the conduction band is where electrons move freely, conducting electricity. The fascinating part? The behavior of these bands, governed by quantum mechanics, dictates whether you can watch TV tonight or not (based on the material used in electronic components!).
Types of Materials Based on Band Structure
- Insulators: These are the hermits of the electron world—electrons stay put in their bands and refuse to conduct electricity.
- Conductors: Think of these as social butterflies. Electrons in conductors move freely and love to conduct electricity.
- Semiconductors: The best of both worlds. Under certain conditions, they can either insulate or conduct electricity, making them stars in electronic devices and solar cells.
Factors Influencing Band Structures
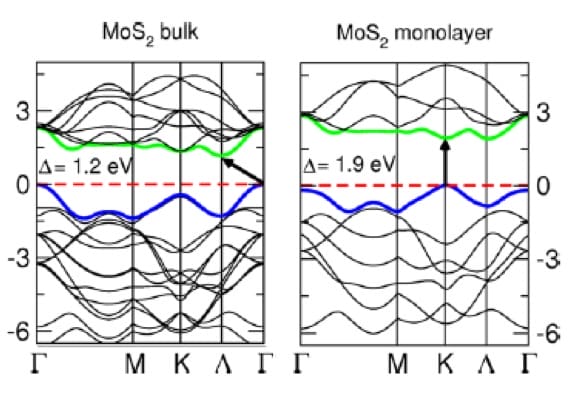
The atomic structure (how atoms are arranged in a material) and electron interactions play significant roles in shaping the band structures. These interactions determine the electronic properties and are why materials behave the way they do under various conditions.
Techniques to Study Band Structures
Scientists use several sophisticated techniques to peek into the electron behavior in materials:
- X-ray Diffraction: It helps us understand the crystal structure that influences band structures.
- Photoelectron Spectroscopy: This technique lets us see the energies electrons occupy within a material.
- Computational Methods: With powerful computers, scientists simulate and predict electronic band structures, saving both time and resources in materials design.
Applications of Band Structure Knowledge
Knowing about band structures enables innovations in multiple fields:
- Electronics and Optoelectronics: From smartphones to LED lights, understanding band structures helps improve these devices.
- Solar Cells: By tweaking band structures, scientists are creating more efficient solar cells.
- Advanced Materials: Aerospace and automotive industries benefit from materials designed with specific band properties for durability and efficiency.
Recent Advances and Research
Recent advancements in the study of electronic band structures are profoundly shaping the field of materials science, particularly through the exploration of two-dimensional materials like graphene and transition metal dichalcogenides. For instance, researchers have developed innovative ways to manipulate band structures actively using external stimuli such as electric fields and mechanical strain. This breakthrough enables the dynamic tuning of material properties, offering tremendous potential for next-generation electronic and photonic devices. Moreover, the integration of machine learning techniques with quantum mechanical modeling has accelerated the discovery and analysis of materials with desirable electronic properties. These computational advancements not only enhance our understanding of band structures but also drastically reduce the time and cost associated with experimental trials. As a result, the ability to predict and engineer specific electronic behaviors in materials is becoming more refined, paving the way for revolutionary applications in technology, from ultrafast transistors to highly efficient solar cells. This trend of marrying computational power with practical experiments represents a significant leap forward, promising to unlock new capabilities in electronics and beyond.
Conclusion
Today, we've scratched the surface of electronic band structures, but this concept is important in materials science, laying the groundwork for countless modern technologies. Whether you're a student, a professional, or just a curious mind, understanding band structures is understanding the building blocks of our material world.
FAQs
Q: Can all materials conduct electricity if they have no band gap? A: Generally, yes. If there's no band gap, electrons can freely move, leading to electrical conductivity.
Q: How do temperature changes affect band structures? A: Temperature can influence electron distribution and mobility, affecting conductivity, especially in semiconductors.
Q: Are there materials with variable band gaps? A: Yes, certain semiconductors can have their band gaps altered through doping or by applying external fields, making them incredibly versatile for different applications.
For help in modelling in any FEA, FDTD, DFT Simulation / Modelling work, you can contact us (bkcademy.in@gmail.com) or in any platform.
Interested to Learn Engineering modelling? Check our Courses?
check out our YouTube channel
u can follow us on social media
Share the resource
-.-.-.-.-.-.-.-.-.().-.-.-.-.-.-.-.-.-
© bkacademy